Kinetic Exclusion Assay
How does it work?
|
Step 1
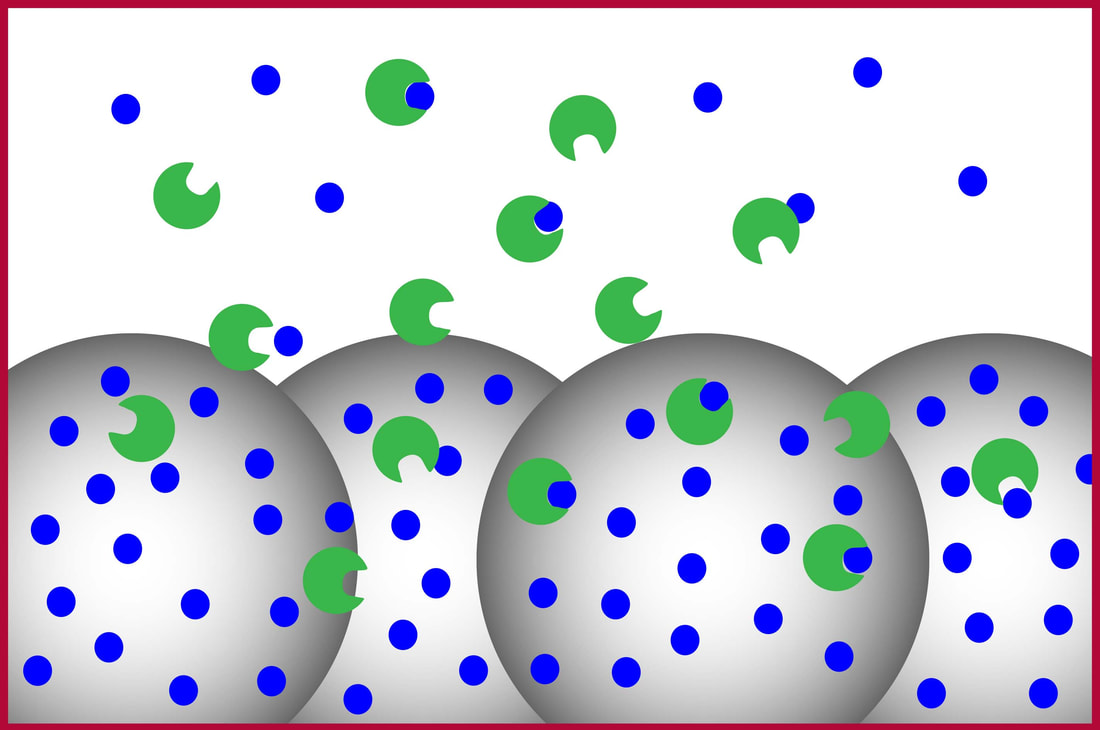
Each mixture quickly flows over beads coated with binding partner B. A small fraction of free binding partner A is captured while complexes are kinetically excluded. |
Step 2
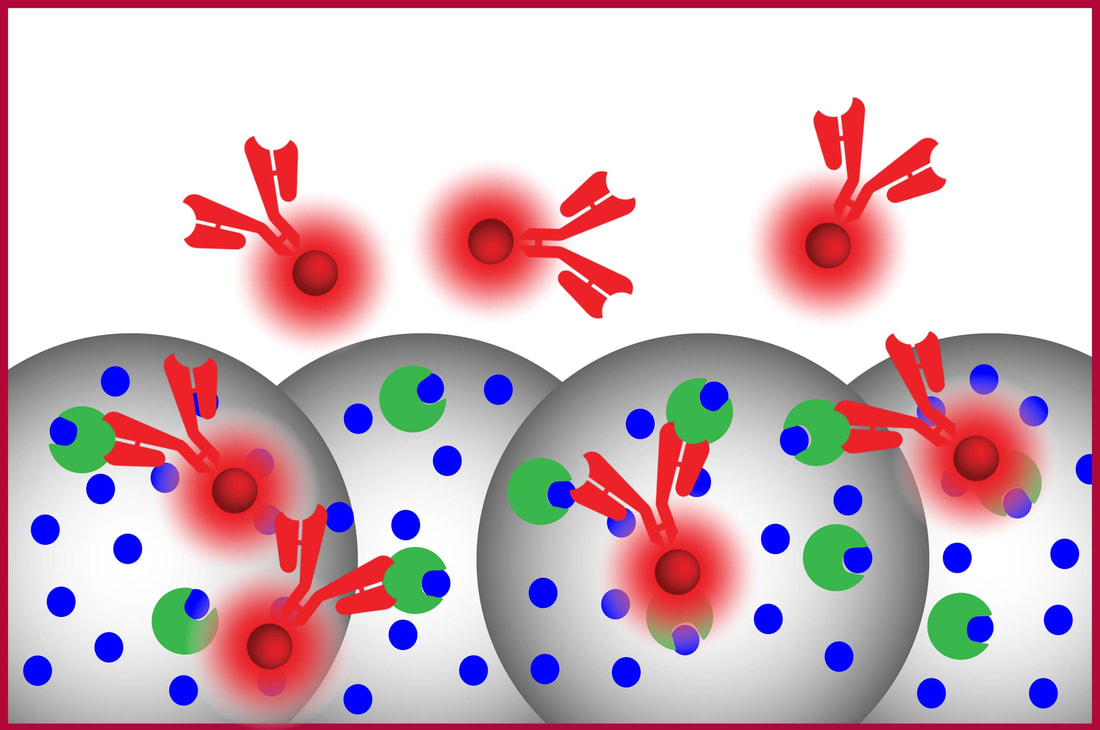
Fluorescently labeled anti-A (label) is introduced |
Step 3
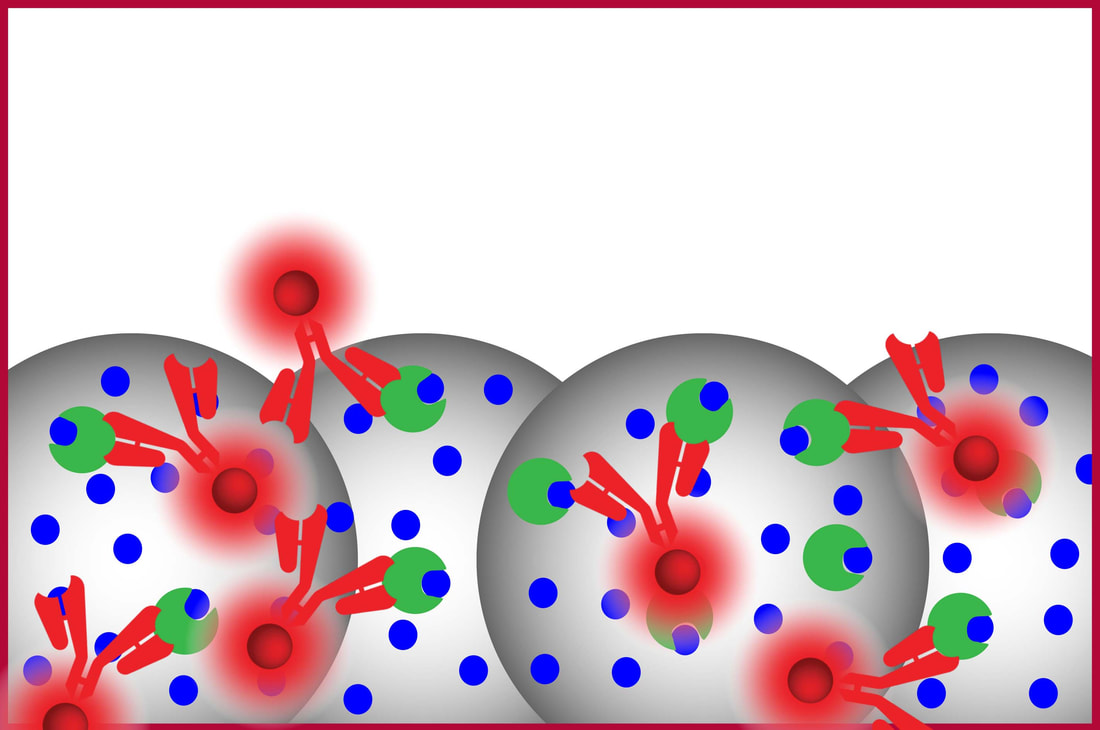
Excess label is rinsed out leaving behind only the label bound to A thus quantifying free A |
What does the data look like?
How does it compare to other biosensors?
KinExA for Affinity
Using a KinExA assay for making Kd measurements is ideal because measurements are made without perturbing the solution equilibrium and with unmodified molecules, unfettered, in solution. For an accurate Kd determination the concentration of receptor in the samples should be near or below the Kd. With the tight binding antibodies (low or sub pM) being developed now, the measurements need to be made at these very low sample concentrations. The KinExA's ability to make quantitative measurements at these low concentrations allow for accurate Kd measurements for those very tight binders.
Binding curves are dependent on Kd and receptor concentration. A binding curve can be generated by making a series of samples with constant receptor concentration and a titration of ligand. After equilibrium is reached, a KinExA measurement is made of the free receptor concentration in each sample of the titration series. Therefore the free receptor directly represents the binding curve. The binding curve generated is then analyzed to find the Kd (for low ratio curves) or receptor active concentration (for high ratio curves). Multiple curves with different receptor concentrations may be analyzed together to get both Kd and active receptor concentration.
KinExA for Kinetics
Measurement of free receptor concentration may also be done prior to the samples reaching equilibrium. Measurements taken as a system approaches equilibrium provide data for the kinetics of the reaction. Two methods can be used for kinetic measurements, the direct method and the inject method.
In the direct method, a single sample of receptor and ligand is prepared and the free fraction is repeatedly measured over time as it approaches equilibrium. The on rate, kon, can be calculated from the curve. This direct measure of the kinetic curve does require that the time the sample takes to reach equilibrium be long enough to allow several measurements to be made. The time of this reaction can be reduced by lowering the concentration of the reactants. The concentration, however, must be kept above the Kd for significant binding to occur. There are situations where weaker binding systems cannot be slowed enough to enable use of this method.
Unlike solid phase kinetic measurements, the molecules in the reaction are unmodified and free to move about in solution. Some differences do occur between the solution phase and solid phase kinetic measurements1 with the solution phase measurement often faster2 – sometimes substantially3.
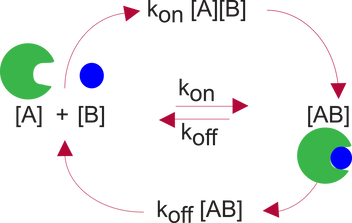
The off rate, koff, can also be directly measured, however it is usually just calculated from the measured Kd and measured kon, (koff = Kd * kon).
2. Razai A., Garcia-Rodriguez C., Lou J., Geren I.N., Forsyth C.M., Robles Y., Tsai R., Smith T.J., Smith L.A., Siegel R.W., Feldhaus M., Marks J.D. 2005. Molecular evolution of antibody affinity for sensitive detection of botulinum neurotoxin type A. J Mol Biol 351: 158-169. http://www.ncbi.nlm.nih.gov/pubmed/16002090
3. Glass T.R., Ohmura N., Saiki H. 2007. Least detectable concentration and dynamic range of three immunoassay systems using the same antibody. Anal Chem 79: 1954-1960. http://www.ncbi.nlm.nih.gov/pubmed/17256970
KinExA for Immunoassay
The ultimate sensitivity of any immunoassay depends on the properties of the antibody used. Most immunoassays do not fully achieve the potential sensitivity of the antibody for two reasons. Either the readout method lacks the sensitivity to fully utilize the antibody's ability or competition is limiting the assay sensitivity. The Kinetic Exclusion Assay (KinExA) prevents competition from interfering, and the measurement sensitivity (sub pM) can take full advantage of extremely tight binding antibodies1.
In practice KinExA has been shown to be 10 to 1000 fold more sensitive than ELISA using the same reagents2. A study was conducted in Japan at the Central Research Institute of the Electric Power Industry (CRIEPI) and funded by the New Energy and Industrial Technology Development Organization (NEDO). The study compared different immunoassay systems by sending identical reagents to outside labs and analyzing the results of the most sensitive immunoassay for each set of reagents. The labs used were Biacore, for an SPR based immunoassay, Sapidyne, for a KinExA based assay, and Kyoto Electronics Manufacturing (KEM) for ELISA. For all 4 sets of reagents the KinExA assay was the most sensitive and had the widest dynamic range3. In fact, KEM has since acquired a license to use KinExA for their commercial dioxin measurement system because of the improved sensitivity, speed, and accuracy compared to ELISA.
2. Blake D.A., Jones R.M., Blake R.C., Pavlov A.R., Darwish I.A., Yu H. 2001. Antibody-based sensors for heavy metal ions. Biosens Bioelectron 16: 799-809. http://www.ncbi.nlm.nih.gov/pubmed/11679258
3. Glass T.R., Ohmura N., Saiki H. 2007. Least detectable concentration and dynamic range of three immunoassay systems using the same antibody. Anal Chem 79: 1959. Figure 4. http://www.ncbi.nlm.nih.gov/pubmed/17256970